
In remote coastal villages where electricity is scarce, a simple yet ingenious solution has emerged to illuminate homes after sunset. Lamps powered not by conventional batteries or grid electricity, but by the very essence of the sea—salt water—are bringing light to communities worldwide. This innovation not only provides a sustainable lighting solution but also showcases human ingenuity in harnessing natural resources for everyday needs.
From classroom science experiments to practical applications in off-grid communities, salt water lamps have captured the imagination of many. They represent a fusion of basic chemistry and a desire for eco-friendly alternatives, offering a glimpse into how simple elements can be combined to solve complex problems.
A salt water lamp works by generating electricity through an electrochemical reaction between metal electrodes submerged in salt water, creating a flow of electrons that powers a light-emitting diode (LED) to produce light.
The Science Behind Salt Water Lamps
At the heart of a salt water lamp is a basic electrochemical cell, akin to a simple battery. When two dissimilar metals, known as electrodes, are placed into a salt water solution, they undergo a chemical reaction that produces an electric current. The salt (sodium chloride) dissolves in water to form positive sodium ions and negative chloride ions, making the water conductive.
One electrode is made of a more reactive metal (like magnesium or aluminum), serving as the anode. The other is made of a less reactive metal (such as copper or carbon), acting as the cathode. The difference in reactivity between these metals causes electrons to flow from the anode to the cathode when connected by a conductive path. This flow of electrons is electricity, which can be harnessed to power an LED light.
The reaction continues as long as the metals are in contact with the electrolyte (salt water) and until the more reactive metal corrodes completely. This process demonstrates the fundamental principles of electrochemistry, converting chemical energy into electrical energy through redox reactions.
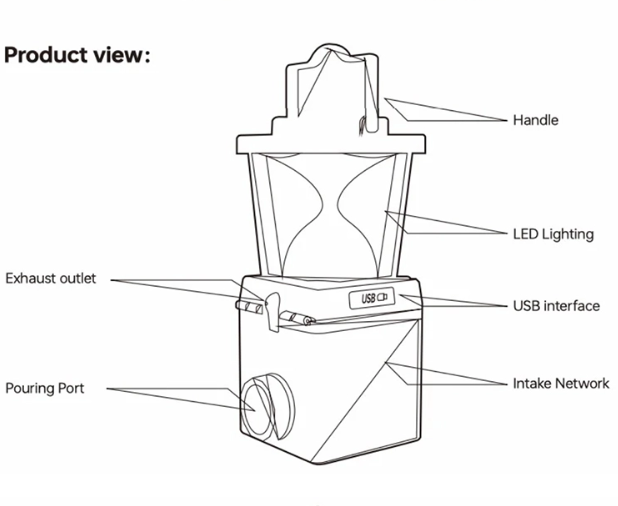
Components of a Salt Water Lamp
Upper Structure:
Handle: Ergonomic design for easy carrying and positioning
LED Lighting: Built-in light source powered by the salt water reaction
Main Body:
Exhaust outlet: Ventilation system for any gases produced
USB interface: Port for potential backup power or charging
Intake Network: Internal system for salt water circulation
Pouring Port: Opening for adding salt water solution
The simplicity of these components makes the salt water lamp accessible and affordable. The electrodes are immersed in the salt water solution, and when connected, the circuit allows electrons to flow, powering the LED. The concentration of the salt solution can affect the efficiency of the lamp; higher salt concentrations generally increase conductivity, improving performance.
Applications and Benefits of Salt Water Lamps
Salt water lamps offer numerous benefits, especially in areas where electricity is unreliable or nonexistent.
Accessibility: The materials required are inexpensive and readily available—salt, water, and metals.
Safety: They do not pose fire risks like kerosene lamps and do not emit harmful fumes, making them safer for indoor use.
Sustainability: Utilizing renewable and abundant resources reduces environmental impact.
Education: These lamps serve as educational tools, demonstrating principles of chemistry and physics.
In disaster-stricken regions or remote areas, salt water lamps provide immediate relief by offering a lighting solution that does not depend on external power sources. They are also beneficial for outdoor activities like camping, where traditional power is unavailable.
Limitations and Challenges
Despite their advantages, salt water lamps have limitations:
Electrode Degradation: The anode metal corrodes over time, requiring replacement. This process can be inconvenient and may generate waste if not properly managed.
Limited Power Output: The electricity generated is minimal, sufficient for small LEDs but not for powering larger devices or appliances.
Maintenance: Regular replenishment of the salt water solution and electrode replacement are necessary to maintain functionality.
Efficiency: Environmental factors, such as temperature and purity of water, can influence performance.
Addressing these challenges is crucial for the broader adoption of salt water lamps. Research into more durable materials and designs aims to extend the lifespan of the electrodes and improve overall efficiency.
Future Prospects of Salt Water Lamp Technology
The potential of salt water lamps extends beyond providing light. Innovations are focusing on:
Improved Materials: Developing new electrode materials that last longer and are more efficient.
Enhanced Designs: Creating lamps that are user-friendly, require less maintenance, and have better energy outputs.
Hybrid Systems: Combining salt water lamps with other renewable energy technologies to increase power availability.
Scalability: Expanding production to reduce costs and make lamps more accessible globally.
Educators and environmentalists see salt water lamps as a gateway to understanding renewable energy and promoting sustainability. By fostering innovation and investment in this technology, salt water lamps could play a significant role in addressing energy poverty and reducing reliance on fossil fuels.
Conclusion
Salt water lamps embody the elegance of applying simple scientific principles to meet essential human needs. By harnessing the natural properties of salt water and metals, these lamps offer a sustainable and practical lighting solution for those without access to electricity.
While challenges remain in improving efficiency and longevity, the continued exploration of salt water lamp technology holds promise. Embracing such renewable energy solutions can contribute to global sustainability efforts and enhance the quality of life in underserved communities. As we illuminate our world with innovative ideas, salt water lamps shine as a beacon of what can be achieved through ingenuity and a commitment to a brighter future.
FAQ
1. How long can a salt water lamp operate before needing maintenance?
A salt water lamp can typically operate for several days to weeks before the anode metal corrodes and needs replacement.
2. Can seawater be used directly in a salt water lamp?
Yes, seawater can be used as it naturally contains salt, but filtering out impurities can help improve performance and longevity.
3. Are salt water lamps safe for indoor use?
Yes, they are safe and do not produce harmful emissions, making them suitable for indoor lighting.
4. What metals are commonly used for the electrodes?
Metals like magnesium or aluminum for the anode and copper or carbon for the cathode are commonly used due to their electrochemical properties.
5. Can salt water lamps power devices other than LEDs?
Due to their low power output, they are generally limited to small devices like LEDs and cannot power larger electronics.
English
العربية
Français
Русский
Español
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
اردو
Afrikaans
Gaeilge
नेपाली
Aymara
Беларуская мова
guarani
Krio we dɛn kɔl Krio
Runasimi
Wikang Tagalog













