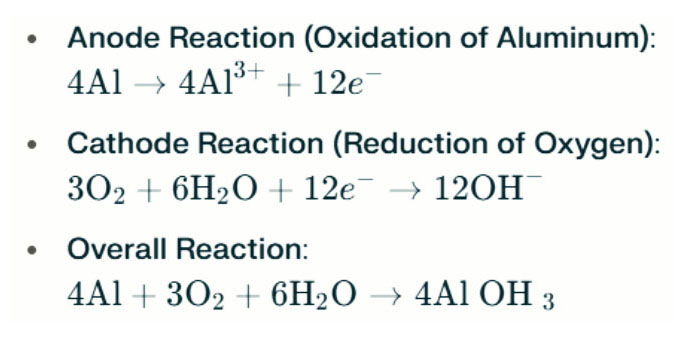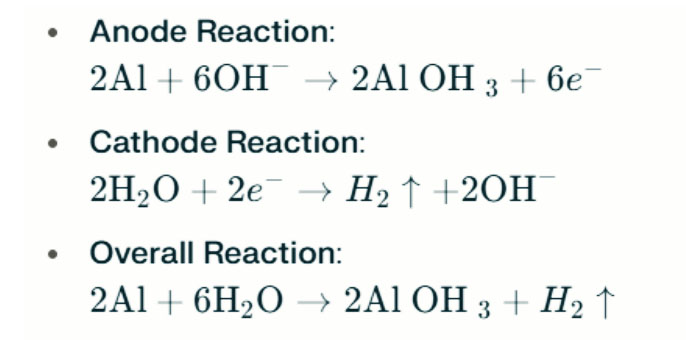Operating Principle
Aluminum-air battery saltwater lamps generate electricity through the electrochemical reaction between aluminum and oxygen in saltwater (the electrolyte). When the aluminum anode contacts the air cathode, it undergoes oxidation, releasing electrons and forming aluminum ions. The chemical reactions involved are as follows:
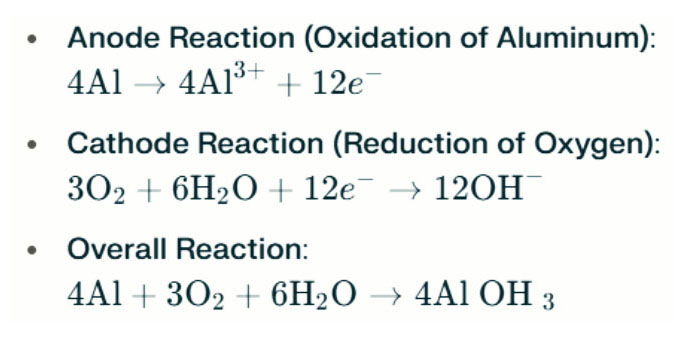
In this reaction, aluminum (Al) reacts with oxygen (O₂) and water (H₂O) to produce aluminum hydroxide (Al(OH)₃), which precipitates as a flocculent solid. Over time, these solid particles may aggregate to form larger crystalline structures. Under suitable conditions, aluminum hydroxide can further dehydrate to become aluminum oxide (Al₂O₃), resulting in a hard solid similar to concrete. This characteristic allows the resulting solid to withstand significant pressure and external forces.
Impact of Using Alkaline Electrolytes
If saltwater is replaced with an alkaline electrolyte (such as sodium hydroxide, NaOH, or potassium hydroxide, KOH), the operating principles and reaction processes will differ. In alkaline environments, the reaction between aluminum and oxygen remains the primary electrochemical reaction. However, due to the alkaline environment reducing the passivation layer on aluminum, its electrochemical activity is enhanced. The reactions in an alkaline electrolyte can be represented as follows:

In this case, aluminum reacts with hydroxide ions (OH⁻) and oxygen to produce aluminum hydroxide ions.
Hydrogen Evolution Phenomenon
In strong alkaline environments, aluminum can undergo hydrogen evolution corrosion, releasing hydrogen gas. The chemical reactions are as follows:
Impact of Using Alkaline Electrolytes
If saltwater is replaced with an alkaline electrolyte (such as sodium hydroxide, NaOH, or potassium hydroxide, KOH), the operating principles and reaction processes will differ. In alkaline environments, the reaction between aluminum and oxygen remains the primary electrochemical reaction. However, due to the alkaline environment reducing the passivation layer on aluminum, its electrochemical activity is enhanced. The reactions in an alkaline electrolyte can be represented as follows:
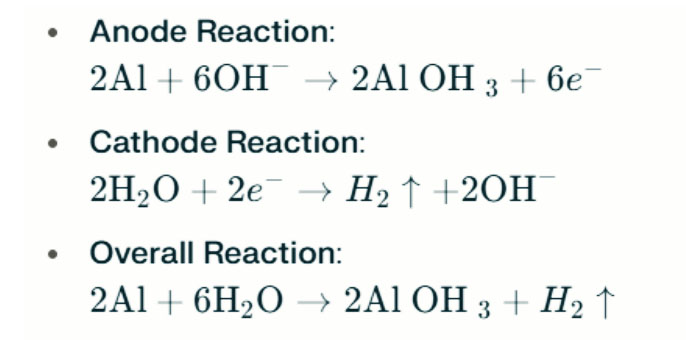
These reactions illustrate the hydrogen evolution process in strong alkaline environments, which can lead to decreased battery performance.
Solid Formation and Hardening
In alkaline environments, the concentration of aluminum hydroxide ions can become high enough for them to precipitate and form solid particles. Over time, these particles may aggregate into larger crystals. The causes of solidification include:
1. Precipitation and Aggregation:
The generated aluminum hydroxide precipitates in the electrolyte and gradually forms larger particles.
2. Dehydration Conversion:
Under certain conditions, aluminum hydroxide may dehydrate and convert into harder aluminum oxide (Al₂O₃), resulting in a very hard solid.
3. Environmental Impact:
The alkaline environment may lead to denser structures in the resulting solid material, enhancing its hardness.
Summary
Thus, while replacing saltwater with an alkaline electrolyte results in similar operating principles and reaction processes, the influence of the alkaline environment on the activity of aluminum metal and hydrogen evolution corrosion can lead to differences in the final solid products and their characteristics. These changes may improve reaction efficiency but also introduce self-corrosion issues.
Usage Considerations for Saltwater Lamps
1. Chemical Reactions:
When saltwater is added, the aluminum plate reacts with oxygen in the air to generate aluminum hydroxide. This compound accumulates over time and forms solid particles that can eventually coalesce into larger masses.
1. Consumption of Aluminum Plates:
If there is a large amount of saltwater, all aluminum plates will be consumed; if there is less saltwater, primarily the lower plates will be consumed. This leads to uneven thickness among the plates.
2. Impact of Solid Particles:
As chemical reactions continue, the accumulation of internal aluminum hydroxide and formation of solid particles will increase pressure on the aluminum plates.
3. Flowability Check:
It is recommended to check the flowability of generated aluminum hydroxide daily. If flowability decreases significantly, replace it with fresh saltwater; otherwise, hardened particles may become difficult to clean.
5. Post-Usage Treatment:
If not used within three hours, immediately empty and rinse out the saltwater lamp's interior, then dry it in a ventilated area while storing it in a cool, dry place.
6. Passivation Phenomenon:
After multiple uses, residual saltwater may not be completely rinsed out, leading to passivation on the surface of the aluminum plates. When new saltwater is added, turn on the lamp first to discharge it; this will disrupt the passivation layer on the plates and restore normal operation.
7. Longevity and Eco-Friendly Features:
Saltwater lamps have a service life of at least 120 hours and can be stored for up to 20 years without requiring additional maintenance during storage. As a new type of environmentally friendly product, they are suitable for use as emergency lighting, teaching tools, or charging electronic devices in various settings such as homes, hotels, firefighting scenarios, fishing trips, hiking adventures, camping excursions, etc. Orders are welcome!
English
العربية
Français
Русский
Español
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ພາສາລາວ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
தமிழ்
Filipino
Bahasa Indonesia
magyar
Română
Čeština
Монгол
қазақ
Српски
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
Հայերեն
עברית
اردو
Afrikaans
Gaeilge
नेपाली
Aymara
Беларуская мова
guarani
Krio we dɛn kɔl Krio
Runasimi
Wikang Tagalog